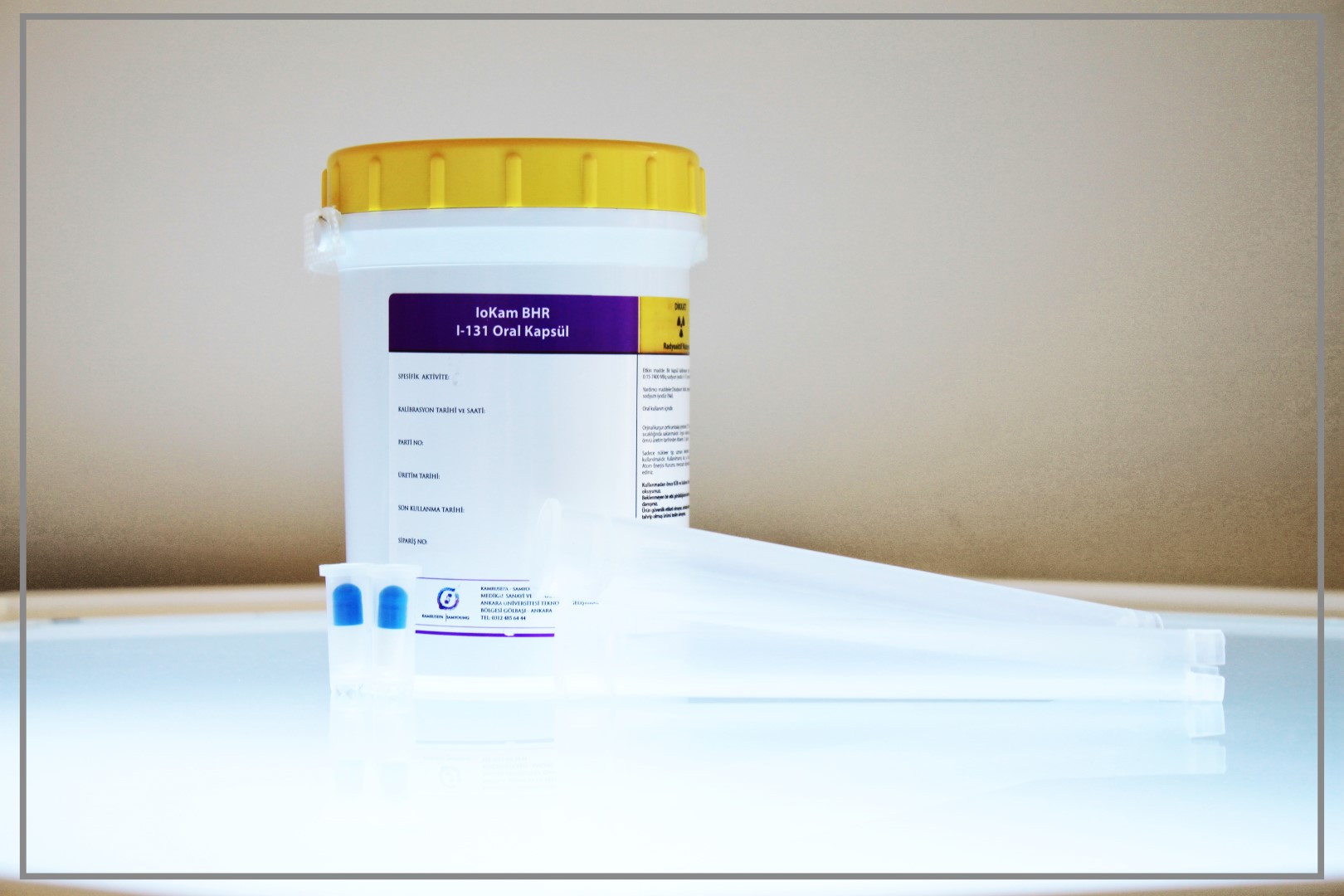
IOKAM BHR I-131 Oral Capsule
Active substance:
Sodium Iodide (131 I): 0.15-7400 MBq / capsule
Additive substances:
Disodium phosphate, water for injection, sodium carbonate-bicarbonate buffer, sodium hydroxide and sodium iodide (Nal).
IoKam BHR I-131 oral capsule in their original lead shield packaging is kept under 25◦C at room temperature in hospitals or application centers.
Oral Capsule will not be used after the expiry date which is stated on the label after “EXP”
It is used for treatment:
Thyroid Cancer,Toxical Nodular Goiter,
Toxical Diffused Goiter Diseases,
Hyperthyroidism
For diagnostic:
Thyroid scintigraphyI-131 Oral Capsulel&Solution Activity Table